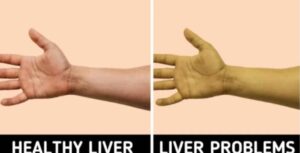
Federal health officials have issued an urgent recall of the widely used antidepressant Duloxetine (Cymbalta, Drizalma Sprinkle, Irenka) after detecting harmful nitrosamine contamination, a probable carcinogen, in delayed-release capsules. The contamination, found during routine testing by Towa Pharmaceutical Europe, exceeds FDA safety limits and affects millions of patients relying on the medication for depression, anxiety, and chronic pain.
The recall underscores systemic issues in pharmaceutical manufacturing, where complex global supply chains and chemical processes can unintentionally produce dangerous compounds. Continues…